Biosimilars vs Generics: What You Need to Know About the Key Differences
When you hear "generic drug," you probably think of a cheaper version of your prescription pill-same active ingredient, same effect, same price cut. But what if your medication isn’t a simple pill at all? What if it’s a complex protein made inside living cells? That’s where biosimilars come in. And here’s the thing: biosimilars are not generics. They’re not even close. Confusing them is like thinking a handmade wooden chair is the same as a plastic folding chair because both let you sit down.
What Are Generics, Really?
Generics are the workhorses of modern medicine. They’re small-molecule drugs-chemicals made in labs, not living cells. Think ibuprofen, metformin, or amoxicillin. Once the patent on the brand-name version expires, any manufacturer can copy the exact chemical structure. The FDA doesn’t need to run new clinical trials. They just need proof that the generic absorbs into your bloodstream at the same rate and to the same level as the original. That’s called bioequivalence. And because the chemistry is simple and repeatable, generics are almost always 40% to 50% cheaper than the brand-name version.There are over 10,000 generic drugs approved in the U.S. today. They make up about 90% of all prescriptions filled. You’ve probably taken one without even knowing it. Your doctor writes a brand name, but the pharmacist hands you the generic. That’s legal in every state. No extra permission needed. It’s automatic.
What Are Biosimilars?
Biosimilars are different. They’re copies of biologic drugs-medications made from living organisms like yeast, bacteria, or animal cells. These aren’t simple molecules. They’re huge, complex proteins, sometimes thousands of times bigger than a typical drug. Examples include Humira (adalimumab), Enbrel (etanercept), and Herceptin (trastuzumab). These are used for conditions like rheumatoid arthritis, Crohn’s disease, and certain cancers.Because they come from living systems, no two batches are exactly alike. Even the original manufacturer can’t make two identical versions. So when a biosimilar is made, it’s not a copy-it’s a highly similar version. The FDA requires manufacturers to prove there are no clinically meaningful differences in safety, purity, or potency. That means hundreds of lab tests, animal studies, and sometimes small clinical trials. It’s expensive. It takes years. And it costs $100 million to $200 million to develop one biosimilar-compared to just $2 million to $5 million for a generic.
Why the Difference Matters
The biggest difference isn’t just cost-it’s how they’re made and how they work in your body.Generics are chemically identical. If you switch from brand-name lisinopril to generic lisinopril, your body can’t tell the difference. Biosimilars, however, have tiny structural variations. These aren’t mistakes-they’re natural outcomes of using living cells to produce proteins. And while those differences don’t usually cause problems, they can trigger immune reactions in rare cases. That’s why switching from a reference biologic to a biosimilar isn’t always automatic. Some doctors prefer to start new patients on biosimilars, but they’re cautious about switching someone already stable on the original drug.
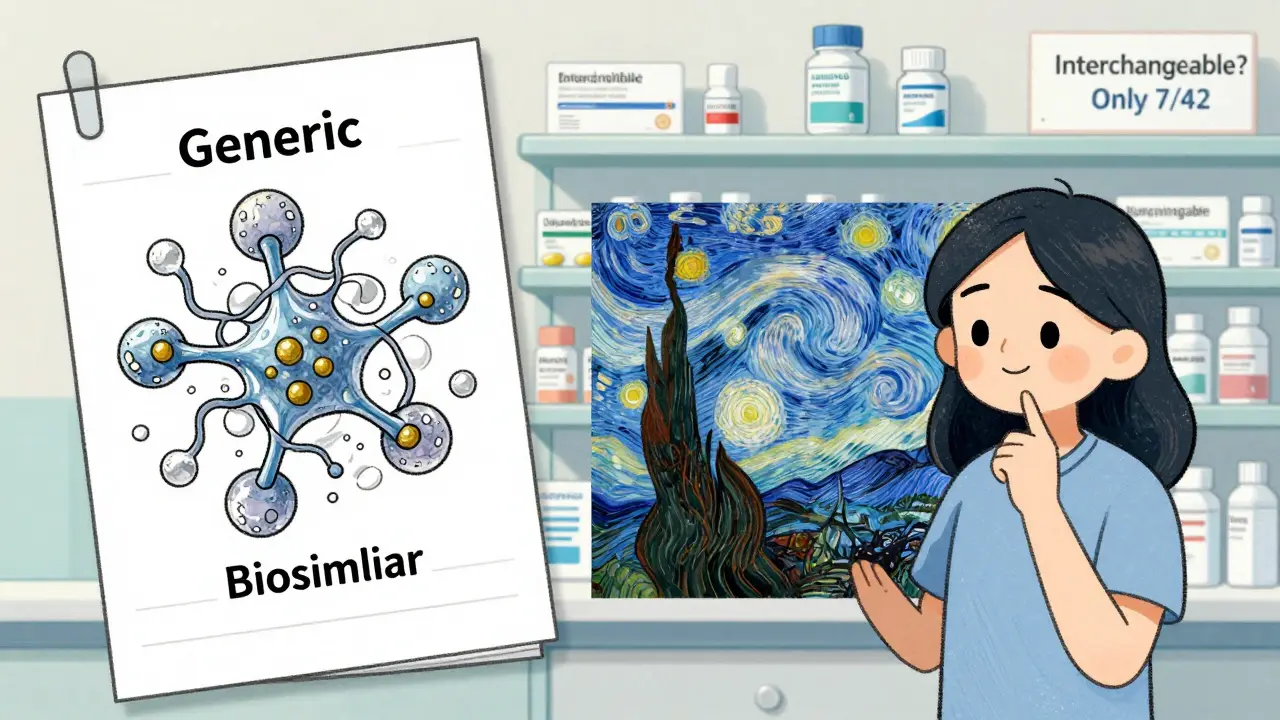
There’s also a legal distinction: only 7 out of the 42 FDA-approved biosimilars as of 2023 have the "interchangeable" designation. That means pharmacies can substitute them without asking your doctor. The rest? You need a new prescription if you want to switch. Generics? No questions asked. The pharmacy can swap them without telling you.
Costs and Savings
Biosimilars save money-but not as much as generics. While generics cut prices by 40% to 50%, biosimilars typically save 15% to 20%. Some, like the interchangeable Humira biosimilar Amjevita, offer up to 35% off. That’s still a big deal when the original drug costs $7,000 a month. But compared to the massive savings from generics, it’s modest.Why the gap? Because biosimilars are harder to make. You can’t just reverse-engineer a biologic like you can a pill. You have to build your own cell line, optimize your fermentation process, and test for dozens of quality attributes. The original manufacturer doesn’t share their secret recipe. So biosimilar companies are playing a high-stakes game of reverse engineering with living cells.
Regulatory Pathways: Two Different Rules
Generics follow the Hatch-Waxman Act of 1984. Biosimilars follow the Biologics Price Competition and Innovation Act (BPCIA) of 2009. These are two entirely different laws with two different standards.Generics: Prove bioequivalence. One or two small studies. Done.
Biosimilars: Prove structural similarity. Test protein folding, sugar attachments, stability, immunogenicity. Animal studies. Pharmacokinetic data. Sometimes clinical trials. It’s a mountain of data.
The FDA’s "Orange Book" lists approved generics. The "Purple Book" lists biologics and biosimilars. The Purple Book is less detailed because the science is too complex to boil down to a simple list. That complexity is why many doctors still don’t feel confident prescribing biosimilars without extra training.
Who’s Using Them and Why
Hospitals and specialty pharmacies are the early adopters of biosimilars. Why? Because they’re buying in bulk for expensive cancer or autoimmune treatments. A 20% discount on a $10,000 drug adds up fast. But in primary care? Generics dominate. They’re cheap, simple, and trusted.Europe is ahead of the U.S. in biosimilar adoption. In countries like Germany and Sweden, biosimilars make up 35% of the biologics market. In the U.S., it’s still under 3%. But that’s changing. Major biologics like Humira, Enbrel, and Stelara are losing patent protection now. More biosimilars are coming. And with the Inflation Reduction Act lowering out-of-pocket costs for biologics, more patients will be able to access them.
Common Misconceptions
Many people call biosimilars "generic biologics." That’s wrong. The FDA, the American Society of Health-System Pharmacists, and top researchers all say the same thing: biosimilars are not generics. They’re a different category.Another myth: if a biosimilar is approved, it’s safe to switch anyone from the original drug. Not true. The American College of Rheumatology says switching should be done with caution and monitoring. If you’ve been on Humira for years and feel great, don’t assume your pharmacist can swap you to a biosimilar without your doctor’s say-so.
And no, biosimilars aren’t "second-rate." They go through more testing than generics. They’re just more complicated. Think of it like this: a generic is a photocopy of a printed page. A biosimilar is a hand-painted replica of a Van Gogh. Same image. Different process. Different result.
What This Means for You
If you’re taking a generic drug, you’re getting a proven, safe, and affordable option. No surprises.If you’re on a biologic-like a shot for psoriasis, rheumatoid arthritis, or cancer-you might soon be offered a biosimilar. Ask your doctor: Is there a biosimilar available? Is it interchangeable? What are the risks and benefits of switching? Don’t assume it’s the same as switching from brand-name to generic.
Don’t let the term "biosimilar" scare you. It’s not a downgrade. It’s a carefully studied alternative. But it’s not a copy. It’s a cousin. And understanding that difference could save you money-and maybe even help you get the right treatment faster.
Looking Ahead
The future of biosimilars is bright. More approvals are coming. More interchangeable products are on the way. The FDA is working on clearer guidance for complex biologics like antibody-drug conjugates. And as more patients and providers get comfortable, prices should drop further.But the road won’t be easy. Patent thickets-like the 240+ patents AbbVie filed on Humira-have delayed competition for years. Insurance companies still sometimes favor the original brand. And some doctors need more education.
Still, the trend is clear: biosimilars are here to stay. They’re not the same as generics. But they’re a powerful tool to make life-changing treatments more affordable. And that’s something everyone can benefit from.
Are biosimilars the same as generics?
No. Generics are chemically identical copies of small-molecule drugs. Biosimilars are highly similar but not identical copies of complex biologic drugs made from living cells. They require more testing and cannot be automatically substituted like generics.
Can pharmacists substitute biosimilars without a doctor’s approval?
Only if the biosimilar has been designated as "interchangeable" by the FDA. As of 2023, only 7 out of 42 approved biosimilars have this status. Most require a new prescription from your doctor to switch. Generics, by contrast, can be substituted automatically in all 50 states.
How much cheaper are biosimilars than brand-name biologics?
Biosimilars typically cost 15% to 20% less than the original biologic, with some interchangeable versions offering up to 35% savings. This is less than the 40% to 50% savings seen with generics, due to the higher cost and complexity of developing biosimilars.
Why are biosimilars more expensive to develop than generics?
Biosimilars are made from living cells, not chemicals. Their structure is complex and variable, requiring hundreds of lab tests, animal studies, and clinical trials to prove similarity. Generics are simple chemical copies that only need to prove bioequivalence through blood absorption studies.
Is it safe to switch from a brand-name biologic to a biosimilar?
For most patients, yes-but it should be done under a doctor’s supervision. The FDA and medical societies agree that switching is generally safe, especially when starting treatment. However, for patients already stable on a biologic, switching carries a small risk of immune reactions. Monitoring is recommended.
What therapeutic areas use biosimilars the most?
Biosimilars are most commonly used in oncology (e.g., trastuzumab for breast cancer), immunology (e.g., adalimumab for rheumatoid arthritis), and endocrinology (e.g., insulin glargine for diabetes). These are high-cost biologics where even modest savings make a big impact.

Maggie Noe
January 9, 2026 AT 05:21Okay but like… if a biosimilar is a hand-painted Van Gogh and the original is the real thing, does that mean my immune system is the art critic? 😅 I just want to not go broke paying for my biologic. Also, can we talk about how wild it is that we treat proteins like they’re coffee machines? 🤯
Aron Veldhuizen
January 10, 2026 AT 06:45You’re all missing the fundamental flaw in this narrative. Biosimilars aren’t ‘similar’-they’re *inherently inferior* by design. The FDA’s ‘no clinically meaningful difference’ standard is a legal fiction. Proteins fold differently. Glycosylation patterns vary. You cannot replicate a living system with chemical precision. This is not science-it’s regulatory theater. And you’re celebrating it like it’s a discount coupon for your life.
Meghan Hammack
January 11, 2026 AT 11:20Hey, if you're on a biologic and scared to switch? Totally get it. But don’t let fear stop you from saving thousands. Talk to your doc, ask if it’s interchangeable, and if they’re unsure? Ask for a referral to a specialist. You’ve got this. 💪 One step at a time, and you’re not alone.
Jenci Spradlin
January 12, 2026 AT 15:44so like… biosimilars are like the fanmade remix of a song while generics are the mp3 copy? idk man i just want my insulin to not cost my firstborn. also why does the purple book even exist? sounds like a middle school art project.
Johanna Baxter
January 14, 2026 AT 07:50Someone’s gonna get sick because some pharmacist swapped their Humira for a biosimilar and now they’re in the ER. I told my cousin this would happen. They didn’t listen. Now she’s on steroids. Again. This is how people die.
Jerian Lewis
January 15, 2026 AT 16:11Generics have been around for decades. Biosimilars? Still experimental in practice. I’m not against savings. But if your doctor hasn’t specifically approved the switch, don’t do it. Your body isn’t a lab rat.
tali murah
January 17, 2026 AT 09:11Let me get this straight: we spent $200 million to create a drug that’s ‘close enough’… and we call that innovation? Meanwhile, generics cost $2 million and are chemically identical. The entire biosimilar industry is a glorified loophole for Big Pharma to extend monopolies under the guise of ‘competition.’
Alicia Hasö
January 18, 2026 AT 09:56To everyone scared of biosimilars: you’re not alone. But please don’t let fear silence your hope. These drugs are rigorously tested. They’re not shortcuts-they’re breakthroughs. If you’re paying $7,000 a month, you deserve better. Ask questions. Demand transparency. And know this: you are worthy of affordable care. This isn’t just medicine-it’s justice.
Micheal Murdoch
January 19, 2026 AT 18:51It’s funny how we treat molecules like they’re sentient. A protein doesn’t care if it was made in a vat of yeast or a Swiss lab. What matters is whether it works. And if a biosimilar does the same job at 1/5 the price, why are we still debating this? The real tragedy isn’t the complexity-it’s that we make patients choose between their health and their rent.
Drew Pearlman
January 19, 2026 AT 19:49Look, I get it. You’ve read the FDA guidelines. You’ve seen the studies. But here’s the thing nobody talks about: doctors don’t understand biosimilars. Most of them learned pharmacology in 2005. They think ‘biologic’ means ‘magic potion.’ And when you tell them a biosimilar is ‘just like a generic but… not really,’ they just nod and write the script for the original. It’s not the science holding us back-it’s the inertia. We need better education, not more regulations. And we need it yesterday. I’ve seen patients cry because they can’t afford their meds. This isn’t theoretical. It’s life or death. And we’re still arguing over semantics.
Chris Kauwe
January 20, 2026 AT 01:11Let’s be real: this is why America’s healthcare system is broken. We outsource drug manufacturing to the lowest bidder, then act shocked when the protein misfolds. Meanwhile, China and Germany are building biosimilar factories like they’re building subway lines. We’re still debating whether a ‘cousin’ is a ‘copy.’ Pathetic. We need national biosimilar standards, not this bureaucratic circus. And stop calling it ‘innovation’-it’s just capitalism with a lab coat.